A metal replaces another metal that is in solution. Up to 24 cash back Single Replacement - Characterized by having an element and compound as reactants and the products are another element and another compound.

Single Displacement Reaction Definition Examples Video Lesson Transcript Study Com
When components of two ionic compounds are swapped two new compounds are formed.
. Orange shirt guy is by himself. Cl 2 KI 5. There are 4 main different types of reactions decomposition with one reactant forming two or more products synthesis with two or more reactants forming one product single replacement with one single-element reactant and one double-element reactant forming another single-element product and another double-element product and double replacement.
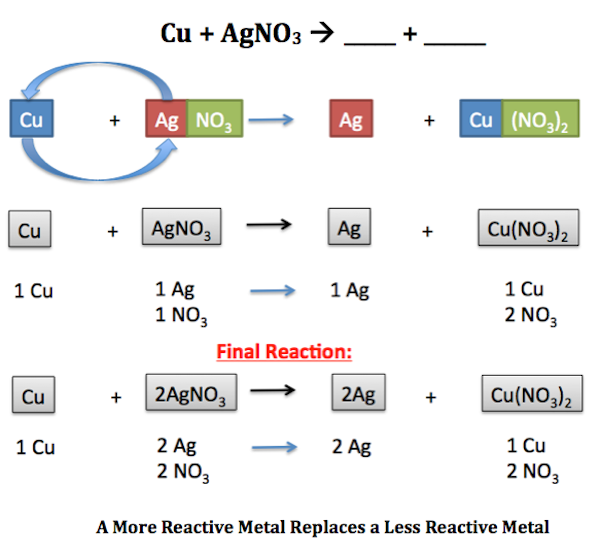
Activity series of metals in Aqueous Solution. 2 K 2 H 2 O 2 K O H H. ZnCuCl2 Cu ZnCl2.
A common single replacement reaction occurs when zinc and copper move from aquous solutions to solids in batteries after electrons to create power. When a single-replacement reaction occurs the element doing the replacing must be _____ than. A change in color A change in odor A change in temperature The production of bubbles.
Neutralization precipitation and gas formation are types of double. Is a chemical reaction in which one element is substituted for another element in a compound generating a new element and a new compound as products. Single Replacement Reactions 1.
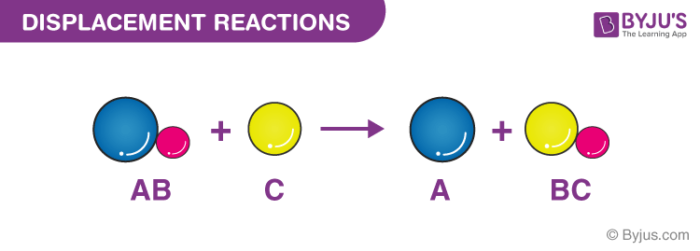
Think about it like these people. A BC B AC Example. In this reaction one cation replaces another one from its solution.
Be sure to Balance each equation. A single replacement reaction also known as a single displacement reaction occurs when one element in a molecule is swapped for another. A reaction in which a single compound breaks down to form two or more simpler substances Decomposition reaction Chemical reaction in which one element replaces another element in.
A metal replaces another metal that is in solution. This can either be in the form of a single replacement reaction or a double replacement reaction. Predicting and determining the products using the reactivity series.
Zinc gives up two electrons and copper gains electrons in single replacement reactions with electricity. There are two types of single replacement reactions. A cation is a positively charged ion or metal.
ABC B AC. If No single replacement reaction occurs write NR to the right of the arrow. Single replacement reactions are chemical reactions where in some cases metals REPLACE other metals that are in a solution.
Double replacement reactions are also called double replacement reactions double displacement reactions or metathesis reactions. Single replacement reaction displacement. In a single replacement reaction one of the reactants is more reactive than the other which results in the formation of a product that is more stable.
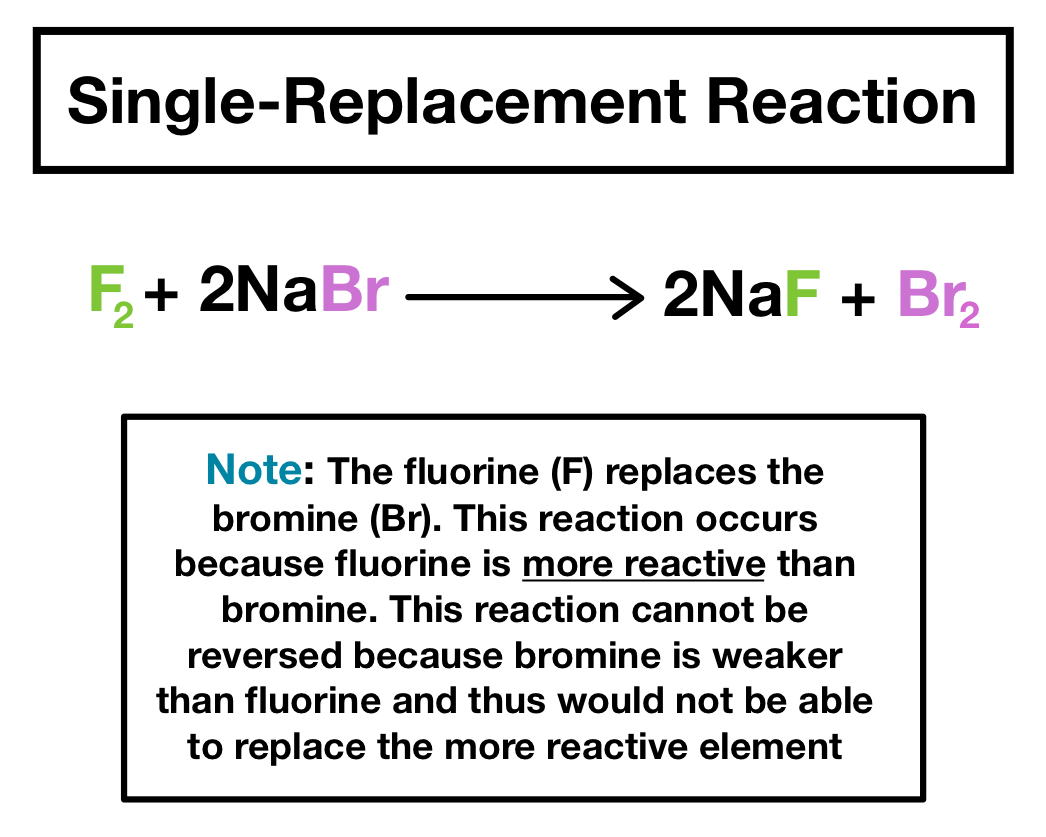
A single-replacement reaction is a chemical reaction in which one element is substituted for another element in a compound generating a new element and a new compound as products. The hydrogen atoms in HCl are replaced by Zn atoms and in the process a new element. In a single replacement reaction a single element replaces an atom in a compound producing a new compound and a pure element.
Al H 2 SO 4 4. A single-displacement reaction also known as a single-replacement reaction is a type of chemical reaction where an element reacts with a compound and takes the place of another element in that. For example 2 HClaq Zns ZnCl 2 aq H 2 g is an.
A single replacement reaction occurs when an element reacts with a compound to produce a new element and a new compound. Definition of single replacement or single displacement reactions. For example 2HCl aq Zn s ZnCl2aq H2g is an example of a single-replacement reaction.
A single replacement reaction occurs when an element reacts with a compound to produce a new element and a new compound. Decomposition reactions happen all around us but we often dont notice them. A single-displacement reaction also called single-replacement reaction is a type of oxidation-reduction chemical reaction when an element or ion moves out of one compound and into another.
The decomposition of carbonic acid in soft drinks which can be represented by the chemical equation H 2 CO 3 H 2 O CO 2. Zn AgNO 3 3. Zn CuCl_2 Cu ZnCl_2 A halogen replaces another halogen that is in solution.
A halogen replaces another halogen that is in solution. All metal displacement reactions are cation replacement reactions. Li H 2.
A replacement reaction is a type of chemical reaction in which one element replaces another in a compound. Using this is helpful when it comes to single replacement. A single replacement reaction occurs when one substance replaces another in a chemical reaction.
A single displacement reaction which is also called as single replacement reaction is a kind of oxidation-reduction chemical reaction when an ion or element moves out of a compound ie one element is replaced by the other in a compound. Which of these does NOT mean a chemical change has occurred. Classification of single displacement reaction.
CHEMISTRY SINGLE REPLACEMENT REACTION WORKSHEET Using the Activity Series Table complete the following reactions by writing the products that are formed. Single Replacement Reactions 2. Some common examples of decomposition reactions are provided below.
Like double replacement reactions metals always replace metals and nonmetals always replace nonmetals in a compound. A double replacement reaction is a type of chemical reaction that occurs when two reactants exchange cations or anions to yield two new products. Alright so were going to talk about single replacement reactions you might also.
Ag KNO 3 2. There are two types of single replacement reactions. Cation replacement reaction A Cation Replacement Reaction.
The electrolysis of water to yield hydrogen and oxygen.
Types Of Chemical Reactions The Science Classroom

Single Displacement Reaction Definition Examples Video Lesson Transcript Study Com
Displacement Reactions Definition Types Single Double Examples
Single Displacement Reaction Definition Examples Video Lesson Transcript Study Com

Single Replacement Reaction Definition And Examples

Single Replacement Reactions Definition Examples Expii

Single Replacement Single Displacement Reaction

Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube
0 comments
Post a Comment